Opioid Treatment With Sublocade in South Carolina
Medically Verified: 2/1/24
Medical Reviewer
Chief Editor

All of the information on this page has been reviewed and verified by a certified addiction professional.
Sublocade is one of the newest FDA-approved prescription medications that is used to treat opioid addiction. When people who suffer from opioid use disorder first stop using drugs, they endure severe flu-like withdrawal symptoms and drug cravings that make it challenging to stay sober. Medications like Sublocade are used in opioid treatment programs in South Carolina to help reduce symptoms of withdrawal and alleviate cravings, making it easier for people to embrace a life in recovery.
What is Sublocade?
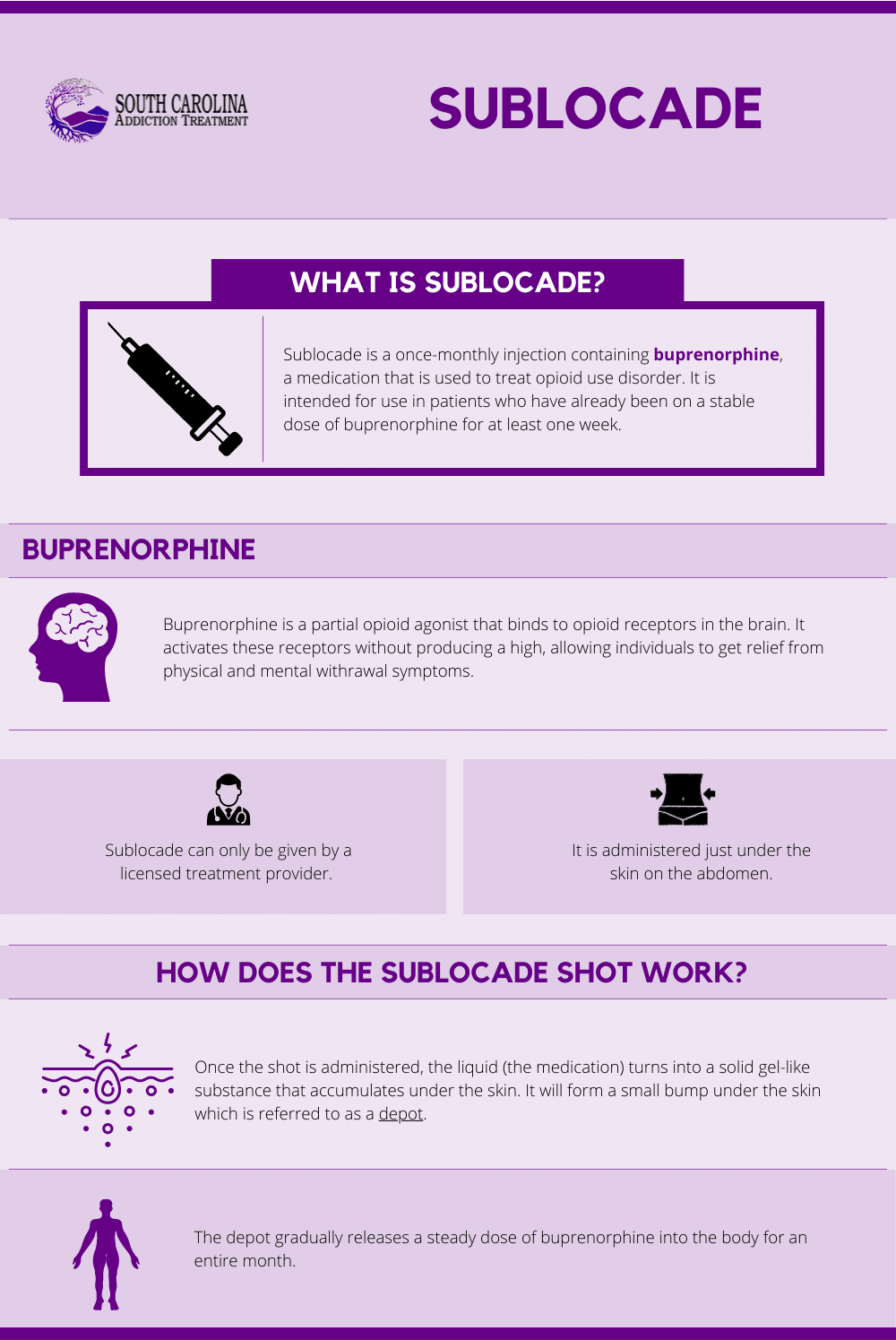
Sublocade was approved by the U.S. Food and Drug Administration in November 2017. It is the first once-monthly injectable buprenorphine medication that has been approved to treat moderate to severe opioid use disorder (OUD). It is intended for use in patients who have already been on a stable dose of buprenorphine for at least one week.[1]
Prior to the FDA’s approval of Sublocade, buprenorphine products were only approved for daily use in the form of a dissolvable strip (Suboxone) or a tablet (Subutex). When combined with an individualized treatment program, buprenorphine products can alleviate opioid withdrawal symptoms and reduce drug cravings.
How Does The Sublocade Shot Work?
Sublocade is a once-monthly injection of buprenorphine. The shot can only be given by a licensed treatment provider. It is administered just under the skin on the abdomen.
Once the shot is administered, the liquid (the medication) turns into a solid gel-like substance that accumulates under the skin. It will form a small bump under the skin which is referred to as a depot. The depot gradually releases a steady dose of buprenorphine into the body for an entire month.[2]
Buprenorphine is a partial opioid agonist-antagonist. This means it binds to and occupies opioid receptors in the brain, but only activates them partially. It is able to provide all of the pain-relieving benefits that opioids do without providing the addictive high and rush of feel-good endorphins. As such, the medication is able to reduce the severity of withdrawal symptoms and cravings for opioid drugs.
Sublocade is intended to be used in combination with a comprehensive treatment program in South Carolina that involves behavioral therapy, counseling, and peer support.
When Can Patients Start Taking Sublocade?
Sublocade can only be given to patients who have already been taking a daily dose of buprenorphine for at least 7 days. This means it usually isn’t administered during the acute phases of opioid detox. Instead, most patients are given an alternative buprenorphine product such as Suboxone or Subutex for one week prior to receiving their first injection. After this period of time, sometimes referred to as the induction period, individuals can begin getting monthly Sublocade injections to help treat opioid addiction.
Side Effects of Sublocade
Sublocade may cause mild to moderate side effects. The most common side effects reported include:[2]
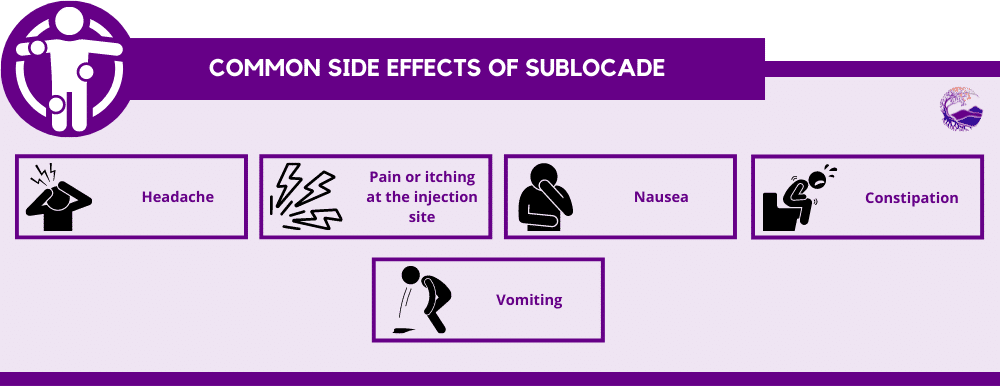
- Headache
- Pain or itching at the injection site
- Nausea
- Constipation
- Vomiting
Other mild, but rare, side effects of Sublocade are:
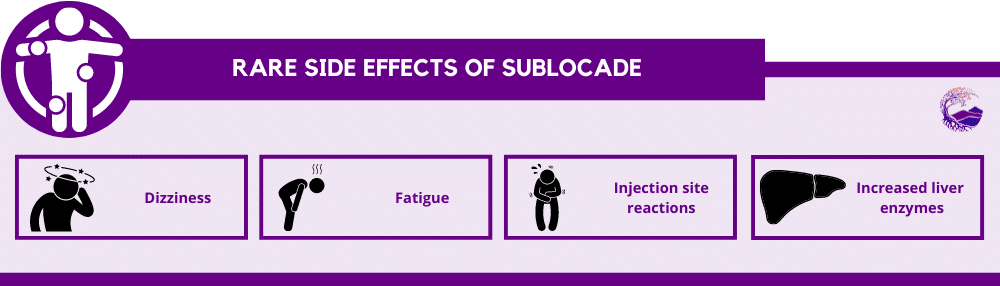
- Dizziness
- Fatigue
- Injection site reactions
- Increased liver enzymes
These side effects are usually temporary and subside after a few days or weeks. However, if they persist, individuals should consult with their physician.
In extremely rare circumstances, Sublocade may cause serious side effects, such as:
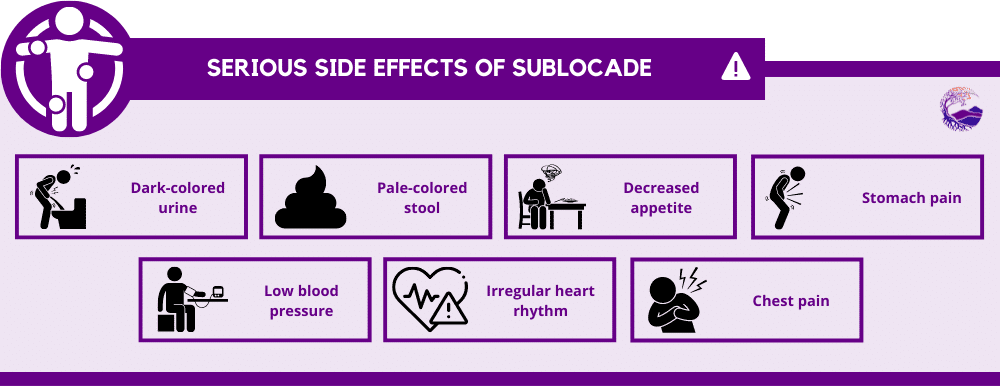
- Dark-colored urine
- Pale-colored stool
- Decreased appetite
- Stomach pain
- Low blood pressure
- Irregular heart rhythm
- Chest pain
Individuals experiencing any of these side effects or any other adverse reaction should seek medical attention immediately. During Sublocade treatment in South Carolina, patients meet with their physician regularly and have other medical staff on-site 24/7 with which they can seek care for serious side effects.
Lastly, if injected into a vein, Sublocade can cause serious harm and even death. This is why the shot must be administered by a licensed healthcare provider.
Medication-Assisted Treatment (MAT) With Sublocade
Medication-assisted treatment (MAT) is an evidence-based approach that combines behavioral therapy and counseling with treatment medications like Sublocade. MAT is thought to provide a more comprehensive and “whole person” approach compared to traditional treatment methods. Since Sublocade does not serve as a stand-alone treatment for addiction, it is often utilized at MAT programs in South Carolina.
During Sublocade treatment, patients have regular visits with their doctor to discuss any side effects they are experiencing and additional concerns. They also have appointments scheduled on a monthly basis for the injection. At the same time, patients may participate in a residential or outpatient program to address the causes behind their addiction as well as healthy coping skills and lifestyle changes that support recovery.
The MAT approach is thought to not only improve treatment outcomes by reducing the rate of relapse but also:[3]
- Improve patient survival
- Increase treatment retention
- Improve patients’ ability to gain and maintain a job
- Reduce rates of HIV or hepatitis C due to drug use
Start Opioid Treatment With Sublocade in South Carolina Today
Sublocade may not be right for everyone. It’s important to speak with a trusted medical provider before starting this or any other medication.
If you or a loved one are addicted to opioids and think treatment with Sublocade in South Carolina is right for you, contact us today. Our trusted admissions counselors can evaluate your needs, verify your insurance, and help you find the right treatment program. Call now to get started.
References:

